Write a Molecular Formula for This Molecule
Write the molecular formulas of the following compounds. Look at the VSEPR chart below to clear your doubts.

Is C3h6o Polar Or Non Polar Acetone Polar How To Find Out Acetone
A condensed structural formula may also be referred to as a semi-structural formula.

. A condensed structural formula is a more compact way of drawing the structural formula of a molecule. While certain basic chemical structures can be suggested by a molecular formula it is not the same as a total chemical structural formulation. This is not particularly difficult as long as you can do simple arithmatic and know the.
According to the VSEPR chart if any molecule has the AX 2 formula then the molecule geometry of that molecule is linear and electron geometry is also linear. For network solids the term formula unit is used in stoichiometric. We were given the molecular weight of the molecule 18018 gmol.
The difference between both is presented. If the molar mass of the compound in problem 3 is 738 gramsmole whats the molecular formula. For example one carbon and two oxygens carbon dioxide.
If the molar mass of the compound in problem 3 is 738 gramsmole whats the molecular formula. Count the number of carbon atoms in the ester molecule. I read your book How to Make a Universe with 92 Ingredients at least three times that fortnight cover-to-cover and I.
Eight years ago aged 10 after forgetting to pack my miniature painting set when I went on holiday my mum bought me a book to cheer me up. The two formulae are related by a whole number ratio such that if the empirical formula is multiplied by the ratio it will yield the molecular formula. Empirical Formula vs Molecular Formula.
You may use the chemical symbol or write out the name of the element. For simple molecules a condensed or. We can also find the electron and molecular geometry of CBr4 using the AXN method and VSEPR chart.
Whats the empirical formula of a molecule containing 187 lithium 163 carbon and 650 oxygen. The molecular formula is a multiple of the empirical formula. Count how many atoms of each element exist in the molecule.
The Relative Molecular Mass of a compound is the sum of the masses of all the atoms present in the molecule. C H O Step 3. It is often shortened to RMM.
So the molecular geometry or shape of the CO2 is linear and its electron geometry is also. A molecular formula enumerates the number of atoms to reflect those in the molecule so that the molecular formula for glucose is C 6 H 12 O 6 rather than the glucose empirical formula which is CH 2 O. Write the molecular formulas of the following compounds.
A compound with an empirical formula of C 2 OH 4 and a molar mass of 88 grams per mole. The molecular geometry of SO2 is bent with a bond angle of 120. What is its molecular formula.
A molecular formula tells us how many atoms of each element make up the molecule. Then count the atoms according to the molecular formula and write them next to the elements name or symbol. 3 Objectives Understand how to write chemical equations Perform calculations based on chemical equations Calculate percent yields from chemical reactions Understand the concept of sequential reactions.
This feels like a good excuse to write to you Ive been meaning to write for a couple years to thank you for your writing. Typically a molecular formula begins with the nonmetal that is closest to the lower left corner of the periodic table except that hydrogen is almost never written first H 2 O is the prominent. It does not talk about the number of atoms of an element present in a molecule.
SO2 is an AX2E type molecule with 2 surrounding atoms ie oxygen and 1 lone pair of. 4 Equations Consider a simple equation. The empirical formula is not the molecular formula.
643 Name each of the following molecular compounds. Instead ionic compounds consist of extremely large collections of cations and anions their relative numbers being in the ratio. However except for very simple substances molecular chemical formulae lack needed structural information and are ambiguous.
Write the formula for the molecular compound iodine pentafluoride. The molecular formula tells you how many of each of those atoms is present in the molecule. Starting from atoms see how many molecules you can build.
First list each element present in the molecule. Potassium phosphide NickelII sulfate Sulfur trioxide. Here A central atom X surrounding atoms and E the lone pairs.
It is not possible to write a molecular formula for ionic compounds because they do not exist as molecules. Divide this number by the molecular weight of the empirical formula to find the number of empirical formula units that make up the compound. CHAPTER 3 Chemical Equations Reaction Stoichiometry.
For example carbon dioxide or CO 2 list that there is 1 carbon C. Write the formula for the molecular compound diboron trioxide. The empirical formula of a compound tells which elements are present in a compound and the relative mass composition of the elements.
Whats the empirical formula of a molecule containing 187 lithium 163 carbon and 650 oxygen. Write the number of of carbon atoms into the skeleton molecular formula as a subscript number to the right of the symbol for carbon C. We can easily find out the molecular geometry of any compound using the given chart.
Identify each of the following compounds as ionic or molecular and give its name. Write a skeleton molecular formula using the symbols for carbon C hydrogen H and oxygen O. For example the molecule acetylene has molecular formula C 2 H 2 but the simplest integer ratio of elements is CH.
A molecular formula is not a chemical name so there are no terms to it. The molecular mass can be calculated from the chemical formula and is expressed in conventional atomic mass units equal to 112 of the mass of a neutral carbon-12 12 C isotope atom. The chemical formulas for covalent compounds are referred to as molecular formulas because these compounds exist as separate discrete molecules.
The RMM is used in many sorts of calculations in chemistry and so you must be able to calculate it to answer all the other calculations you might meet. So the AXN generic formula for the CO2 molecule becomes AX 2 N 0 or AX 2. Collect your molecules and view them in 3D.
In a condensed structural formula or semi-structural formula covalent bonds are not always. AXN is a simple formula that represents the number of the bonded atom and lone pair on the central atom to predict the shape of the molecule using the VSEPR chart. A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole.
AXN notation for CBr4 molecule.

4 2b Writing A Chemical Formula Given A Molecular Model Youtube

How Do You Write Chemical Formula For An Ionic Compound Wedding Jewellery Inspiration Delicate Jewelry Necklace Antique Jewelry Necklace

Is Becl2 Polar Or Nonpolar Beryllium Chloride Chemical Formula Molecules Biology
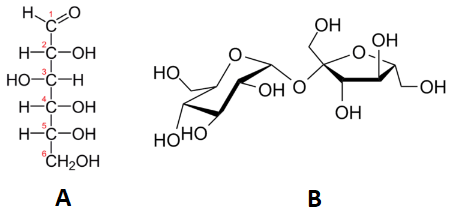
6 9 Calculating Molecular Formulas For Compounds Chemistry Libretexts

Is No2 Nitronium Ion Polar Or Nonpolar Nitrogen Dioxide Polar Molecules

3d Penicillin G Molecule Molecules Penicillin Organic Molecules
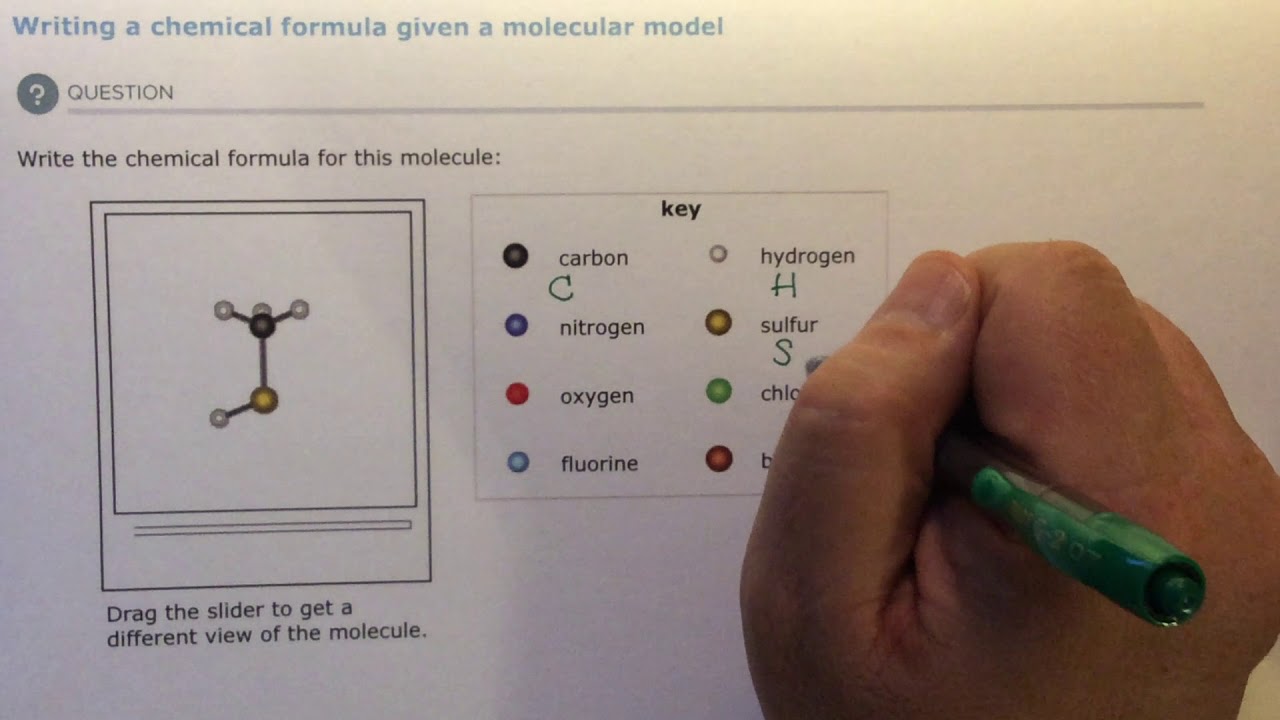
Aleks Writing A Chemical Formula Given A Molecular Model Youtube

Ch2cl2 Lewis Structure Dichloromethane Molecules Hydrogen Atom Lewis

In This Video We Are Going To Determine The Polarity Of Dihydrogen Sulfide In This Molecule It Is Made Up Of Hydrogen And S Molecules Chemical Formula Biology

Sucrose Molecule 3d Model Website Template Design 3d Model Molecules

Difference Between Empirical And Molecular Formula Infographic Chemistry Lessons Chemistry Study Guide Chemistry Education

Is Sf2 Polar Or Nonpolar Sulfur Difluoride Polar Chemical Formula Home Decor Decals

Clf3 Molecular Geometry Bond Angles Electron Geometry Molecular Geometry Molecular Molecules

Is Ch3cl Polar Or Nonpolar Methyl Chloride Methylation Hydrogen Atom Molecules

No2 Lewis Structure Nitrogen Dioxide Nitrogen Dioxide Molecules Lewis

Pin By Qkmaqcba On Communications Logos Negative Space In 2022 Molecule Model Communication Logo Chemical Formula

Chemical Formula Chemistry Basics Chemistry Lessons Science Notes

Is Pcl3 Polar Or Non Polar Phosphorus Trichloride Phosphorus Chemical Formula Molecules
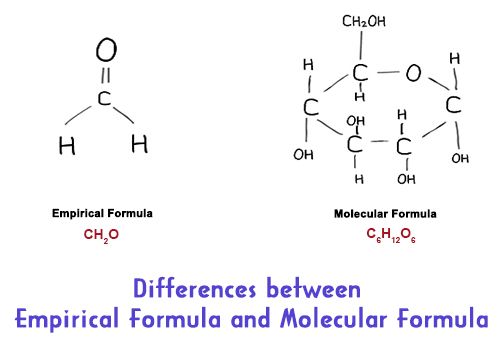
Differences Between Empirical Formula And Molecular Formula Qs Study
Comments
Post a Comment